When we get this question, it’s typically assumed that the customer thinks we only fill our dampers with nitrogen gas. We get this question at shows, even though we have our clear damper models that people can try for themselves and literally see the damper oil inside the damper.
We in fact do use nitrogen gas, but the damper’s internal volume also comprises of damper oil as well. Whether it is a twin tube or monotube setup, they both use damper oil and nitrogen.
The damper oil, which is a specific weight and proprietary for our use, is what helps us regulate damping force. Our shims, which are used to control oil flow through the piston valve (and base valve, if applicable), are matched with this weight oil and it makes our engineers’ jobs easier when determining valving specifications for each application or for revalved dampers.
The oil also helps to transfer kinetic energy (and friction) from the the damper’s operation into heat energy, which is then dissipated through the damper body and radiated into the air.
Nitrogen gas is filled to a certain pressure, determined when we’ve calculated how much damper oil each damper is to receive (and how much the piston shaft will displace during compression). Nitrogen gas does several things. For one, it helps to allow the piston shaft to compress into the damper. If, for example, we only filled the damper with oil and we filled it completely (with no air gaps), the piston shaft wouldn’t compress into the damper at all. This is because oil is incompressible.
The piston shaft requires a certain volume within the damper. Think of it as going into a pool that’s filled to the brim with water. When you go in, you will displace some of that water. No matter how slowly you enter the pool, the water will want to spill out. The piston shaft wants to do the same exact thing, pushing oil out of the damper. But since it is fully sealed, there’s nowhere for the oil to go, so the piston shaft can’t compress.
Nitrogen is compressible, on the other hand. If we fill the remaining internal volume with nitrogen gas (at a specific pressure), the piston shaft will now have room to compress into the damper. I mentioned that the gas has to be at a specific pressure. Too much pressure and the shaft will basically have a hard time compressing into the damper. Again, because gas still has mass, it has to be a controlled amount that you pressurize the damper to. This effectively creates damper rebound. Since the piston shaft can compress into the damper in one direction, the piston rod can rebound in the opposite direction. Now we have a damper that offers control for both compression and rebound, and the shim stacks on the piston valve help control the speed of the piston valve for a given velocity.
Nitrogen also has some distinct advantages than using air. Air contains mostly Nitrogen by volume (depending on the altitude, that may change slightly), but because air also comprises of oxygen and hydrogen, there’s a chance that moisture can form within the damper. We use nitrogen because it helps reduce aeration and cavitation during damper operation. Very high piston speeds can create cavitation, which translates into a momentary loss in damping force. Aeration is the formation of bubbles in the damper oil, which also affects damping force. If we used just air as the remaining volume in the damper, these problems will be exacerbated. Using only nitrogen helps reduce these potential problems.
Nitrogen also expands at a constant rate compared to just air. That means a more consistent internal pressure as the damper operates. Because it also has a greater density than just air, that means a damper can hold its pressurized charge for a longer period of time.
One other characteristic is that nitrogen is inert. It’s safe to use for shock absorbers. It will not react to other components of the damper, even the damper oil. Keep in mind, however, that shock absorbers are pressurized. The nitrogen may be inert, but the damper oil can be flammable (which is why we warn end-users of exposures of shock absorbers to open flames).
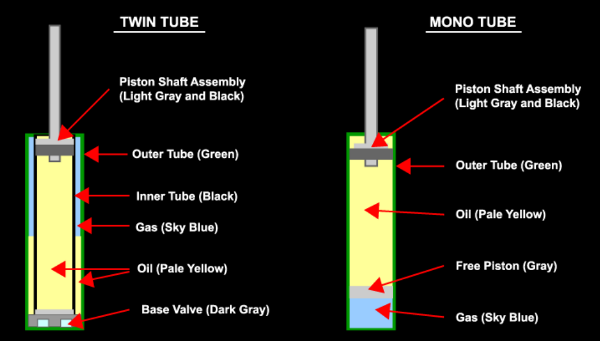
In a monotube damper, the nitrogen charge is separated by a floating (or “free”) piston. That means that the oil is completely separate of the nitrogen. Since the floating piston is free moving, as the piston shaft compresses into the damper, the floating piston will compress the nitrogen charge.
On a twin tube damper, the oil and nitrogen share the same volume. While the inner tube, or working tube, of the damper is basically all damper oil, the outer tube is a mix of some of the damper oil while the top (because the gas is lighter than damper oil) is nitrogen. So, as the piston shaft compresses into the damper, the oil is physically compressing the oil in the outer tube.
There are a few issues that can exist with a twin tube damper. For one, it works best when near vertical alignment and with the base valve towards the bottom. Again, because nitrogen is lighter, it’s tendency is to rise to the highest point inside the damper. But, the base valve can only work properly in damper oil, otherwise it will lose damping effectiveness. This is why you never really see an inverted twin tube setup, unless you were to have one of our HG dampers (which you can read about The Pains of Rally Racing), then it’s technically possible. Although best in a near vertical alignment, you can position a twin tube at a few degrees from vertical, so long as the nitrogen doesn’t have a chance of entering the working tube. However, if nitrogen were to enter the working tube, it can be worked out into the outer tube during function. But, while it is still in the working tube, it can create a gap in damping force until the gas eventually works its way into the outer tube again.
There are other inert gases that can technically be used, but nitrogen’s density is far greater and meets the purposes of a damper’s function pretty well.
So, there you have it. We use nitrogen AND oil in our dampers.